Minerals are extraordinary creations of nature, each possessing its own unique set of properties and characteristics. From their chemical composition to their crystalline structure, minerals have captivated the minds of mineralogists, gemmologists, and crystal enthusiasts for centuries. Learning about crystal systems can support you in learning the principles of mineralogy from a scientific point of view. In this blog post, we will delve into the fascinating world of crystal systems, which provide a framework for classifying crystals based on their symmetry and internal geometry.
This is a complementary article to our Mineral and Gem Properties Guidebook which you can download for free here.
Table of Contents
What are Minerals?
Before we look into what are the distinguishing markers of crystal systems, let’s go over what minerals are. Minerals are naturally occurring inorganic substances that have a specific chemical composition and a distinct crystalline structure. They form through various geological processes over millions of years. These exquisite formations can be found in different geological settings, at the surface of the Earth or deep within its crust. The diversity of geological environments contributes to the wide variety of minerals found on Earth, each with its own story of formation and transformation.
 The shape of Crystals : Crystalline Structures and Atomic Arrangements
The shape of Crystals : Crystalline Structures and Atomic Arrangements
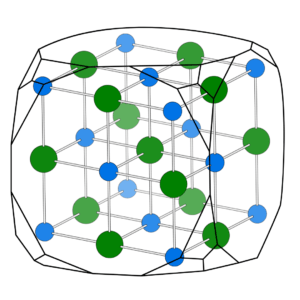
Crystals grow in very specific ways. They have what is called a crystalline structure, an ordered arrangement of atoms or ions within a three dimensional lattice. This internal arrangement gives minerals their distinct geometric shapes and symmetry.
Different atoms, meaning different chemical elements, combine to form unique mineral species with their own distinct physical and chemical attributes. The periodic table and the understanding of chemical composition are thus directly correlated to the shape of crystals, and crystal systems.
How crystals form and grow: chemical elements, pressure and temperature
Crystals form under specific conditions involving temperature, pressure, and availability of certain elements. These vary depending on the geological environment. Different crystals require different combinations of factors to grow.
Chemical composition is directly related to formation of crystals. For instance, quartz, one of the most abundant minerals on Earth, is composed of two types of atoms: silicon and oxygen. These atoms are arranged in a specific repeating pattern, known as a crystal lattice. This structure is responsible for quartz’s characteristic properties (more on mineral properties in our free guidebook).
 It is also interesting to note that minerals with similar compositions can form different structures depending on temperature and pressure. For example, both diamond and graphite are composed of carbon atoms, but their distinct structures result in vastly different properties: diamond is incredibly hard and transparent, while graphite is soft and opaque.
It is also interesting to note that minerals with similar compositions can form different structures depending on temperature and pressure. For example, both diamond and graphite are composed of carbon atoms, but their distinct structures result in vastly different properties: diamond is incredibly hard and transparent, while graphite is soft and opaque.
Understanding the role of chemical composition is essential in identifying and classifying minerals, and understanding how minerals are classified in crystal systems.
What are Crystal Systems?
Now that we’ve addressed what are minerals and how they form, we can look at the different categories in which they are organized.
Crystal systems are a way of classifying crystals based on their symmetry and geometry. There are seven crystal systems: cubic, tetragonal, hexagonal, trigonal, orthorhombic, monoclinic, and triclinic. Essentially, crystal systems describe the internal arrangement of atoms in a crystal, which dictates its external shape. Think of it as the blueprint for how a crystal is constructed from the inside out.
 Why Learn about Crystal Systems?
Why Learn about Crystal Systems?
Identifying minerals can be an exciting challenge for mineral enthusiasts. Physical properties such as color, hardness, luster, and cleavage can provide clues to a mineral’s identity. However, to gain a deeper understanding, we turn to crystal systems.
By observing the symmetry of a crystal and measuring its angles, we are able to narrow down the possibilities when it comes to identifying a crystal. This identification process can sometimes be done simply by observation, but it is important to note that it actually has to do with the internal axis of the crystal. Understanding crystal systems is key in identifying and classifying minerals.
The Seven Crystal Systems
Each crystal system is characterized by a particular type of symmetry and has a unique set of geometric properties.
Cubic System
 The cubic system is one of the most symmetrical and includes some of the most iconic gemstones like diamonds and garnets. In this system, all three axes are equal in length and intersect at right angles (90 degrees).
The cubic system is one of the most symmetrical and includes some of the most iconic gemstones like diamonds and garnets. In this system, all three axes are equal in length and intersect at right angles (90 degrees).
- Symmetry: High symmetry with multiple planes.
- Unique Traits: Often forms in shapes like cubes, octahedrons, and dodecahedrons.
- Mineral Examples: pyrite, fluorite, halite, galena, diamond
Tetragonal System
 In the tetragonal system, two axes are equal in length while the third is different; all axes intersect at right angles.
In the tetragonal system, two axes are equal in length while the third is different; all axes intersect at right angles.
- Symmetry: Moderate symmetry with fewer planes than cubic.
- Unique Traits: Commonly forms prismatic crystals with square cross-sections.
- Mineral Examples: vesuvianite, apophyllite, wulfenite, scheelite, zircon, cassiterite
Hexagonal System
 The hexagonal system features four axes: three are equal in length and lie in a plane intersecting at 120 degrees, while the fourth axis is perpendicular to this plane.
The hexagonal system features four axes: three are equal in length and lie in a plane intersecting at 120 degrees, while the fourth axis is perpendicular to this plane.
- Symmetry: High symmetry around one axis.
- Unique Traits: Forms hexagon-shaped prisms or pyramids.
- Mineral Examples: quartz, beryl (such as aquamarine or emerald), apatite, graphite, corundum (including gemstones such as ruby and sapphire).
Trigonal System
 Often considered a subset of the hexagonal system due to similar characteristics but distinct enough to be classified separately, trigonal crystals have three equal axes intersecting at 120 degrees with a fourth axis that differs in length.
Often considered a subset of the hexagonal system due to similar characteristics but distinct enough to be classified separately, trigonal crystals have three equal axes intersecting at 120 degrees with a fourth axis that differs in length.
- Symmetry: Similar to hexagonal but less symmetrical overall.
- Unique Traits: Typically forms rhombohedral or trigonal prisms.
- Mineral Examples: tourmaline, calcite, hematite, dolomite, magnesite
Orthorhombic System
 This system has three unequal axes that all intersect at right angles.
This system has three unequal axes that all intersect at right angles.
- Symmetry: Lower symmetry compared to cubic and tetragonal systemss.
- Unique Traits: Often forms elongated or tabular crystals.
- Mineral Examples: Topaz, olivine, sulfur, aragonite, staurolite.
Monoclinic System
 In monoclinic crystals, two axes intersect at an angle other than 90 degrees while the third remains perpendicular to them both.
In monoclinic crystals, two axes intersect at an angle other than 90 degrees while the third remains perpendicular to them both.
- Symmetry: Even lower symmetry with only one plane of symmetry.
- Unique Traits: Frequently forms prismatic or bladed crystals.
- Mineral Examples: Gypsum, muscovite, orthoclase feldspar, augite, clinopyroxene.
Triclinic System
 The triclinic system has no right-angle intersections between its three unequal axes.
The triclinic system has no right-angle intersections between its three unequal axes.
- Symmetry: Lowest symmetry among all crystal systems.
- Unique Traits: Rarely forms simple shapes; often appears as distorted or asymmetrical crystals.
- Mineral Examples: asinine, rhodonite, kyanite, microcline, plagioclase feldspar, turquoise.
Mineral Anomalies: When Crystals Break the Rules
While the seven crystal systems provide a great framework for understanding crystal structures, nature loves exceptions! Mineral anomalies can occur due to impurities, variations in temperature and pressure during formation, or structural defects within the crystal lattice itself. This can potentially make identification harder, but gives us all sorts of interesting mineral particularities to take into account when developing a mineral collection. In fact… you may, like me, develop a particular interest in mineral anomalies.
Let’s look at examples of how these anomalies can impact crystal formation:
Impurities
Impurities can introduce foreign elements into a crystal’s structure during its growth process, altering its appearance and structure. For instance:
- Color Variations: Trace amounts of elements like iron, manganese, or chromium can change the color of a mineral. For example, pure quartz is clear, but the presence of iron can give it a purple hue, creating amethyst. This color change might lead to confusion in identifying the crystal system if the observer relies solely on color as a distinguishing factor.
- Shape Distortions: Impurities can disrupt the regular atomic arrangement, causing irregularities in crystal faces and angles. These distortions can make it difficult to recognize the typical geometric shapes associated with a specific crystal system.
Temperature and Pressure Variations
Different conditions during mineral formation can cause deviations from standard crystal structures. In different temperatures or pressure, crystal shapes may be drastically altered. Higher temperatures might lead to larger crystal growth with more pronounced facets. Extreme pressures can compress atoms more tightly together, resulting in denser crystals with altered geometries. For example:
- Polymorphism: Carbon can form diamond or graphite depending on the pressure and temperature during its formation. Diamonds form under high pressure and high temperature, while graphite forms under lower pressure and temperature conditions. Identifying the crystal system might be challenging if the mineral exists in a transitional state or has mixed characteristics.
- Crystal Twinning: Changes in temperature and pressure can cause crystals to twin, where two or more crystals share some of the same crystal lattice points. This can complicate the determination of the crystal system as the twinned crystals may not exhibit the typical symmetry or shape of their crystal system.
Structural Defects
Defects within the atomic lattice—such as vacancies where an atom is missing or dislocations where atoms are misaligned—can also affect a crystal’s overall structure. These imperfections may create unique visual effects like iridescence or play-of-color.
Defects within the crystal lattice, such as dislocations, vacancies, and interstitials, can influence the shape and properties of the crystal. For example:
- Dislocations: Line defects within the crystal lattice can cause the crystal to deform and grow in unusual shapes. This can make it difficult to match the crystal to a specific system based on its external morphology.
- Vacancies and Interstitials: Missing atoms (vacancies) or extra atoms (interstitials) can distort the crystal lattice, leading to irregular growth patterns. Such anomalies can obscure the characteristic geometric features of the crystal system.
In summary, mineral anomalies can obscure the typical features of a crystal system, making it more challenging to accurately classify the crystal. Understanding these anomalies and their effects on crystal growth is crucial for correctly identifying and studying minerals.
Identifying Crystal Systems in Your Collection
 For those passionate about minerals and crystals, building a collection can be an incredibly rewarding experience. Here are some tips to develop your knowledge base as you grow your collection, by determining which system a particular mineral belongs to.
For those passionate about minerals and crystals, building a collection can be an incredibly rewarding experience. Here are some tips to develop your knowledge base as you grow your collection, by determining which system a particular mineral belongs to.
- Examine External Shape: While not always definitive due to external factors affecting growth patterns (like space constraints), observing general shapes (cubes vs hexagons) provides initial clues about possible classifications.
-
Observe Symmetry Planes: Pay attention when rotating specimens under light sources; consistent reflections indicate higher symmetries typical among certain groups (e.g., cubic vs triclinic).
-
Measure Angles: If you are able to measure the angles, using tools like goniometers, you can measure angles between faces accurately—a critical step since different systems feature distinctive angular relationships among their axes!
- Consult guides/manuals: Acquiring a general guidebook on mineralogy can be a great way to learn the basics of mineralogy. When you acquire a specimen, you can consult mineralogy books, or even simply the internet, to develop your knowledge of the mineral in question
- Consult your local mineral club, or mineralogy facebook groups:Connect with other enthusiasts or join local mineral clubs to expand your knowledge and share experiences. Different mineralogy facebook groups or forums can be a great way to validate information, get multiple opinions, and develop your knowledge base.
- Maintain a Mineralogy Logbook: Whether you use one of our mineral collection logbooks or your own notebook, keeping track of the information you learn about your specimens will ensure that you build your knowledge base over time.
By combining these techniques and research methods, you’ll gain deeper insights into each specimen’s intrinsic beauty rooted firmly within its respective crystalline framework!
Conclusion: Developing your interest in Mineralogy with sound Foundations
 In conclusion, crystal systems provide a valuable framework for understanding the internal geometry and symmetry of minerals. By studying these systems, we gain insights into the formation processes, identify minerals, and appreciate their unique beauty. Whether you are a seasoned mineral enthusiast or just beginning your journey, the world of crystals awaits your exploration. So dive in, discover the wonders of crystal systems, and let the magic of minerals captivate your imagination.
In conclusion, crystal systems provide a valuable framework for understanding the internal geometry and symmetry of minerals. By studying these systems, we gain insights into the formation processes, identify minerals, and appreciate their unique beauty. Whether you are a seasoned mineral enthusiast or just beginning your journey, the world of crystals awaits your exploration. So dive in, discover the wonders of crystal systems, and let the magic of minerals captivate your imagination.
You can get your hands on our free Mineral and Gem Properties Guide here.